Main market for surfactants is the personal and home care industry, as they belong to the most important ingredients of cleaning products. However, they are also used in food industry as emulsifiers, in biochemical applications for solubilization, in polymer industry for stress tests, as additives in lubricants, in fire-fighting applications, and many more. The liquid detergent market value alone is project to reach USD 40 million within the next few years. The increasing demand for environmentally friendly and natural products will be a driving force in the detergent market entailing the need for appropriate product development.
The Challenge
Surfactant molecules which possess a polar head and a nonpolar tail part are known as amphiphiles and they spontaneously aggregate to form association structures such as micelles, bilayers and vesicles in aqueous solution. Determining the phases and the structural transitions of the micelle is very important for predicting the washing and cleaning mechanisms of surfactants. The measurement of phase behaviors and characterization of various micelle structures are difficult and time-consuming. Since there is a growing interest in finding natural and environmentally compatible replacements with equal or even better performance, molecular modeling can be utilized reasonably for efficient and smart product development.
The Work
For studying the behavior of surfactant mesostructures, non-equilibrium Dissipative Particle Dynamics (DPD) simulations using the MAPS/LAMMPS DPD plugin have been performed. This approach allows to simulate appropriate time and length scales of this class of phenomena. In this case study, we compare the effect of two different surfactants, the ionic Sodium Dodecyl Sulfate (SDS) and the non-ionic Cocamide surfactant. Both surfactants are commonly used in detergent formulations and we simulate the effect of removal of an oil film from a cellulose surface.
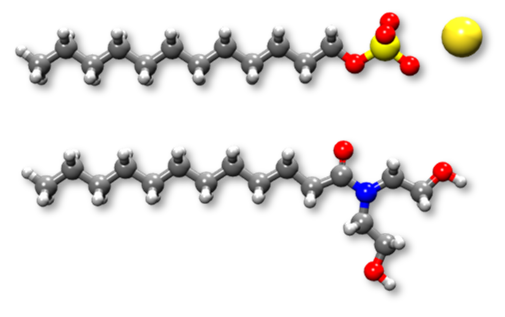
Figure 1: Atomistic representation of surfactants: Top: sodium dodecyl sulfate, bottom: lauramide diethanolamine (major component of cocamide).
The Results
The effect of surfactants on a layer of oil on a Cellulose surface has been studied. For this purpose, mesoscale suitable models have been developed. The phase behavior and micelle formation has been simulated for one ionic and one non-ionic surfactant. The simulations revealed that the ionic SDS surfactant allows diffusion of the oil/surfactant micella through the system, whereas in the case of the non-ionic Cocamide surfactant the micelle stays static on the cellulose surface and is thus less suited for washing power formulations.
The presented pseudo-experimental approach is ideally suited for investigating phenomena related to micelle formation and phase behavior. Moreover, applications like drug delivery and release, can be addressed in a similar fashion. In this way, simulations can support product design efficiently and avoid costly experiments.
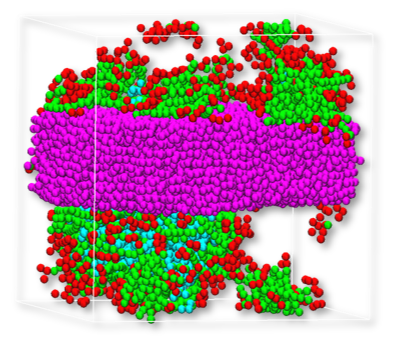
Figure 2: Snapshot from DPD trajectory after equilibration. Left: sodium dodecyl sulfate, right: cocamide. Color code: cellulose – purple, oil – blue, surfactant – red and green.
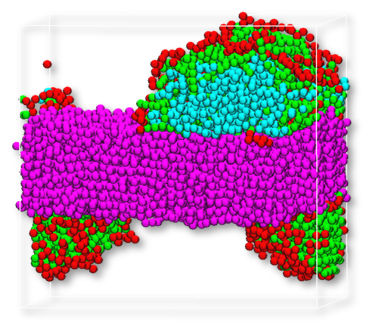
Figure 2: Snapshot from DPD trajectory after equilibration. Left: sodium dodecyl sulfate, right: cocamide. Color code: cellulose – purple, oil – blue, surfactant – red and green.
