In a multitude of chemical processes separation of mixtures is a critical step. One prominent example is the purification of reaction products. In particular, removal of phenol is of great industrial importance. With a global production of 8 million tons each year in 2001, phenol is one of the most important intermediates in chemical industry. It plays a central role in the production processes of bisphenol A, phenol formaldehyde resins, or the Hock process, but also in waste-water treatment.
The Challenge
Technical separation methods are extremely important in order to reach high-quality products in acceptable yields at low costs. In this context, knowing the phase behavior and thermodynamic properties is a vital aspect for chemical engineering. For generating a large amount of data over a wide pressure, temperature and composition range, fast and reliable simulation methods are essential. Computational methods, such thermodynamic models combined with Monte Carlo simulations are ideally suited for this purpose and can assist in predicting the thermodynamic properties of industrially used chemicals, like for example phenol and its mixture with water, which is relevant for waste-water treatment and phenol recycling.
The Work
Equation of state modeling based on PC-SAFT (perturbed chain statistical associated fluid theory) as implemented in the MAPS SciTherm module has been used to calculate the binary phase diagram of the water/phenol mixture. The MAPS Towhee plugin was used to perform Monte Carlo (MC) simulations to generate additional data for refining the PC-SAFT prediction.
The Results
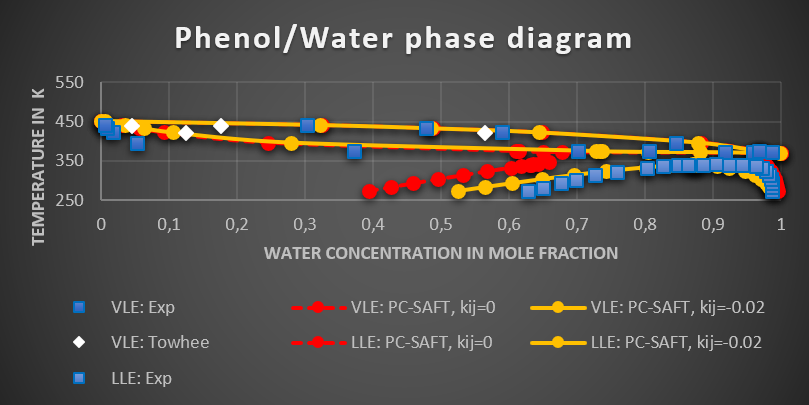
The critical temperature of Phenol was calculated using the density scaling law fit and the boiling point determined from the Clausius-Clapeyron equation with the calculated saturated vapor pressure. A combination of Monte Carlo simulations and thermodynamic equation of state was used to predict the phase equilibrium of the industrially important phenol/water mixture. Figure 1 shows the experimental phase diagram, and the PC-SAFT predicted phase diagram before and after refining the parameters using Monte Carlo simulations. Also, the Monte Carlo predictions are included in the diagram. It can be clearly seen, that the quality of the predictions improves dramatically, when the pseudo- experimental data from Monte Carlo simulations is included.
These kind of simulations are important to know for chemical process engineering. In this way, modeling helps to focus the required experiments and becomes an alternative if poisonous or costrly chemicals are involved.